Understanding the migration thresholds in printing inks
Considering the minute amounts of substance which can lead to non compliance, examination of the individual material combinations in inks and their migration risk is indispensable. In this article we focus on the migration thresholds in the USA and Europe markets.
28 Apr 2017 | By WhatPackaging? Team
Positive list principle
For direct food contact materials, in particular for plastics, there are regulations in place which demand that only those substances be used in the manufacture of these materials that are approved for this purpose (positive list principle). In many cases, the affected industry compiles comprehensive toxicological data on substances of interest to obtain this approval.
The evaluation of substances is performed by toxicologists of the authorities. In case of EU legislation it is done by the experts from the European Food Safety Authority (EFSA) based on a very demanding and comprehensive set of data on toxicity focusing on the chronic effects from lifelong exposure to the substances in question.
Migration (EU): Depending on the quality of the toxicological data, acceptable exposure levels from food intake (to which the consumer can be exposed lifelong) are determined with high safety margins. The legislator compiles these final toxicological evaluations in the positive lists which are part of the packaging regulations. Thus these positive lists provide the data for acceptable transfers into food for each individual substance (specific migration limit, SML). While many substances are restricted by an SML, it should be understood that for those substances without an SML the food packaging regulations specify an upper limit for the substance transfer, which is defined as the sum limit of all substances migrating into food (overall migration limit, OML).
Migration (USA): In the USA, the positive lists for food contact materials issued by the Food and Drug Administration (FDA) are equivalently based on very demanding industry notifications and toxicological evaluations. The substances listed therein are authorised (having been ‘evaluated’) for the respective food contact materials and, in many cases, are subject to further limitations such as maximum use concentrations which shall safeguard that migration will be lower than quantities of concern. As well, the overall migration is limited.
Printing inks
While in Europe (except for Switzerland – and Germany in the future) and in the USA, as well as in almost all other parts of the world, printing inks on food packaging are currently not regulated via positive lists, it is by coincidence that many evaluated substances for food contact materials are also in current use for printing inks. According to the European Plastics Regulation, if evaluated substances are contained in a printing ink printed on plastic packaging, the entire packaging, including the printed layer, must comply with these limits. In addition, the OML (in Europe normally 60 mg/kg food, equivalent to 60 ppm), will also be applied for the entire packaging, including the printed layers. Both provisions require attention from the ink manufacturer and the converter.
Non-evaluated substances
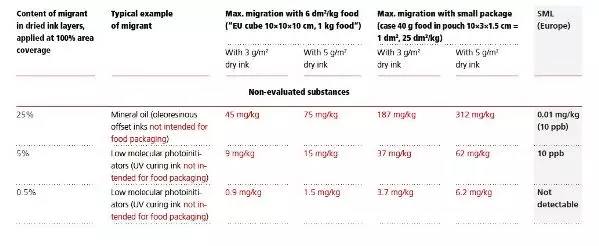
All substances which are used in printing ink formulations but which are not officially approved by a national authority, and thus not explicitly and positively listed as evaluated in regulations and guidelines, are principally to be regarded as ‘non-evaluated’. It is common sense amongst legislators worldwide that substances without or with only a small amount of toxicological data are not considered safe, by default. Relevant regulations in Europe, Switzerland, and in the USA determine that non-evaluated substances should not be detectable in food. It is important to be aware that this requirement is compulsory, and it is enforceable by food control authorities.
Europe: There is a general understanding in Europe – and in Switzerland an undisputable legal provision (and the same will hold true for Germany in the future) – that ‘not detectable’ means that a default migration threshold of 10 μg/kg (= 10 ppb) for all of these substances is applicable. However, for substances with carcinogenic and/or mutagenic properties, the legally enforceable threshold will be lower, i.e. correspond to the most sensitive, reliable analytical method available.
USA: In the USA, although not backed up by the FDA’s official consent, there has long since been a general consensus in the scientific community that, for claiming absence of migration of non-approved substances, a default detection limit of 50 ppb is, as a rule, deemed sufficient. However, control authorities are entitled to assess on a case-by-case basis at which concentration in food a non-approved substance would be tolerated, respectively at which action level an enforcement measure would be imposed. As outlined above, this threshold can only be ignored if favourable toxicological data supports a higher safety margin. The resulting new threshold would have to be determined by recognised toxicologists and according to the criteria established by EFSA for Europe, or respectively by the FDA for the USA.
Acceptable migration is very low
The packaging chain should be aware of the minute amounts of substances which can, in case of migration, lead to noncompliance:
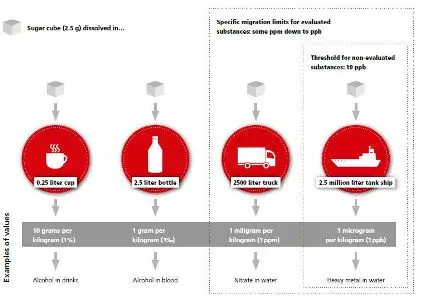
Migration assessment via worst-case calculation
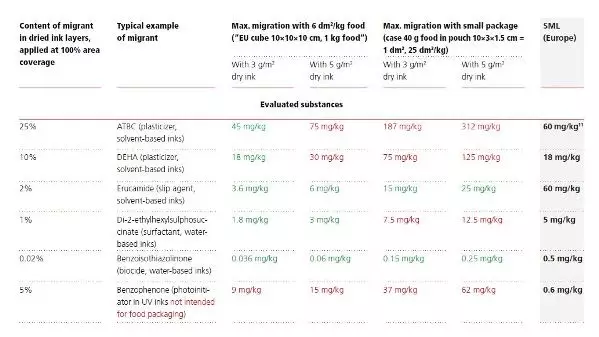
The following table gives an idea about the maximum amounts that can theoretically migrate into food from the printed layers. These calculations are based on ‘100% migration’ also known as ‘worst-case’ migration.
These worst-case calculations are immediately applicable in Europe, Mercosur and China. Of course, migration into conventionally packed food is not likely to occur to this maximum extent as the majority of practically observed migration cases do not even come close to the worst-case assumptions. This is substantiated by public studies and many observations made in industries which indicate that under normal conditions only a minor or even a minuscule part does actually migrate into the foodstuff.
However, regulations stipulate that this assumption must be verified on the packaging in its finished state (or by software-aided migration modelling).
If the quantity of all potential migrants in all components of a certain food packaging is known, the so-called ‘worst-case’ calculation is a reliable method to verify the maximum migration possible. Siegwerk has a ‘worst-case calculator’ available for customers on www.Siegwerk.com/en/customersegments/customer-service/food-packaging-safety.
European regulations explicitly allow the verification of compliance via this method. If the results of the worst-case calculation for the packed food unit are lower than the applicable thresholds, no further measures, such as practical migration testing, are required.
However, it must be noted that the permissible migration is not stipulated for average packed food in average packaging; in fact, authority control labs ultimately examine actual packed food in the actual packaging unit. Therefore, all potential factors of influence, such as the ratio of surface to volume of food and the other parameters have to be carefully considered. In case of any doubt, the real migration should be determined by the printer and the food packer using official analytical methods stipulated by the regulations.
It is to be noted that, depending on the nature of the packed food unit and the individual migrant, the European regulations provide certain additional checks of migration results (normally conversions which are alleviations) such as, for some migrants, multiplication with a ‘fat reduction factor FRF’ before comparison with legal limits, or application of a default surface/volume ratio (EU cube) instead of the actual one in case of low volume packaging. Particular attention is required with regard to the verification of compliance of non-evaluated substances, as the following table demonstrates.

This is the second of the four-part article series on printing inks for food packaging by Siegwerk.














 See All
See All